Integrating physics and biology
Blending microfluidics and stem cell biology, our C-Stem™ technology generates alginate capsules seeded with induced pluripotent stem cells (iPSCs) and is engineered to mimic the in vivo stem cell niche.
A single platform to overcome the critical challenges of Cell & Gene therapy
C-Stem™ is a proprietary platform that integrates a GMP encapsulation technology, effectively addressing several key challenges in the cell therapy industry - producing cell therapies of high-quality, at scale, efficiently. This pioneering technology achieved a world-first in 2021 by delivering 15 billion cells in a single batch. A distinctive attribute of C-Stem™ is its capability to amplify and differentiate cells within a closed system, thereby enabling enhanced quality control. It has the potential to change the landscape of cell therapy development.


Integrating physics and biology
Blending microfluidics and stem cell biology, our C-Stem™ technology generates alginate capsules seeded with induced pluripotent stem cells (iPSCs) and is engineered to mimic the in vivo stem cell niche.

Little capsules, great benefits
The capsule technology protects the cells, allowing them to expand and differentiate in large-scale bioreactors without suffering from impeller-induced shear stress and having equal access to nutrients.

A unique 3D format for efficacy
The result is a 3D microtissue format, that allows for enhanced quality control and functionality improvement, favoring engraftment.
Addressing bottlenecks
The breakthrough technology addresses critical challenges in the industry of scale and quality, in addition to efficacy and safety of future cell therapy products. We address these challenges to develop innovative treatments for diseases such as Parkinson's Disease.
Scalability
Our C-Stem™ technology allows for the production of stem cells at an unprecedented scale without changing key parameters, opening up new possibilities for research and therapeutic applications.
One run produces up to 15 billion iPS cells.
Safety & Quality
With our C-Stem™ technology, you can expect preserved genomic integrity and yield mature cell phenotypes, enhancing the safety of cell therapy products.
Efficacy
The C-Stem™ technology delivers precise and consistent results, guaranteeing consistent quality and performance in every batch. We deliver functional microtissues that integrate well by graft and reduce time-to-effect.
Revolutionary
At TreeFrog Therapeutics, we use a single platform to amplify and differentiate iPSCs into ready-to-transplant 3D format microtissues, with proprietary devices.


Industrial-grade microfluidics
GMP encapsulation device
Automated, closed system (class B/ISO 6 Compatible)
High stem cell encapsulation throughput
> 1000 capsules a second

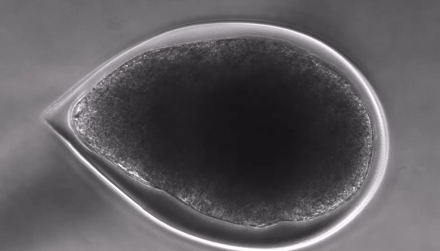
Biomimetic 3D micro-environment
Cells self-organize in-vivo like stem cell microtissues
Epiblast sructure promotes fast cell division in a protected environment letting nutrients in and out.


Bioreactor scale-up
Exponential stem cells expansion & differentiation in bioreactors
> 277 fold hPSCs amplification per week
> 98% cell viability
> 97% pluripotency & genomic integrity preserved
Single-batches of +15 billion cells in 10L bioreactor

Quality ensured
Development of proprietary imaging devices and imaging techniques to ensure highest quality of 3D microtissues
Proprietary imaging system for automated capsule quality check
In-process capsule quality control

Easy downstream processing & delivery
Easy capsule removal in minutes to release cell content
Mature cell phenotypes enhance product safety & efficacy
3D microtissue format facilitates handling & delivery
;)
;)
Opportunities
We believe in #Celltherapyforall and as such, we are pursuing not only our own programs, but we want to partner with the best in other therapeutic areas to bring cell therapies to patients as soon as possible.
We are open to licensing and co-development opportunities in other therapeutic areas and can provide

From 2D to 3D
Our 3D stem cell biology team with a dedicated R&D platform can adapt existing 2D protocols to C-Stem™ for optimal hPSCs amplification and differentiation.


Scale-up early
Our bioproduction team can develop bioreactor-based manufacturing processes to secure commercial-scale batches for non-clinical and early clinical phases.


Enhance delivery
Our team of scientists brings expertise in logistics, delivery formats and medical devices to maximize the clinical convenience and therapeutic benefits of the product.


Optimize manufacturing
Following tech transfer to a partner site, our encapsulation and analytical development teams provide continuous support for the optimization of manufacturing processes.
Evolution
C-Stem™ is the result of over 10 years of research collaboration and 5 years of intense work within TreeFrog by stem cell biologists, biophysicists, engineers and pharma development teams. Checkout the milestones so far!
2013
2018
Development of the C-Stem™ Technology within the academic sphere
Biophysicist Kévin Alessandri and Stem Cell Biologist Maxime Feyeux, met and worked together to develop a 3D print encapsulation system in 2015. Together with leading academics and PhD teams, the invention of the biomimetic C-Stem™ technology that TreeFrog Therapeutics is based upon was developed.
Checkout some of the founding publications;)
;)
Kévin Alessandri
;)
;)
Maxime Feyeux
2018
TreeFrog Therapeutics is established.
2018
The teams kick-off of the R&D encapsulation device prototype development in collaboration with Invetech.
2020
In march 2020, TreeFrog receives the first prototype encapsulation system with a throughput of 1,000 capsules per second.
2021
TreeFrog achieves a World First producing 15 billion cells in 10L bioreactors (a x277 fold expansion).
2022
In april 2022, TreeFrog receives the first GMP encapsulation system.
Read the press release2023
In april 2023, the first encapsulation data is published in Biomaterials "Engineering 3D micro-compartments for highly efficient and scale-independent expansion of human pluripotent stem cells in bioreactors" by Philippe Cohen et al.
Read Phillippe Cohen’s paper2024
Transfer of our first GMP encapsulation device to a Contract Development Manufacturing Organisation (CDMO) takes place.
Reproducibility batches of our Parkinson's Disease product are produced.